Chlorine dioxide (ClO2) treatment can be a safe and effective solution for preoxidation and disinfection. In our previous webinar, “A Competitive Review of Preoxidants,” we compared several common chemistries used in a disinfection program. Overall, chlorine dioxide was found to be the most well-rounded of the preoxidants with the consideration that each of the chemistries could be used as part of a complete multi-barrier treatment program.
In this article, you will learn the most common uses of chlorine dioxide and how to implement ClO2 into your drinking water treatment program.

Common Uses of Chlorine Dioxide
We most commonly see utilities with high TOC source water adding or switching to chlorine dioxide in their treatment programs to reduce chlorine-derived disinfection byproducts (DBPs) such as Trihalomethanes (THM) and Haloacetic acids (HAA5). This, of course, is regulatory driven to comply with the Stage 2 Disinfectants & Disinfection Byproducts Rule (EPA Office of Water, 2010).
The second most common need is oxidation of naturally occurring iron and manganese from source water to enhance removal in settling and filtration and to improve water aesthetics. ClO2 tends to excel here because of its faster reaction times while simultaneously reducing the risk of exceeding DBP values as compared to the higher concentrations of chlorine required.
We also see growing interest in using chlorine dioxide in primary disinfection to obtain log inactivation credits to satisfy the Long Term 2 Enhanced Surface Water Treatment Rule (EPA Office of Water, 2006). Chlorine dioxide is a much more potent disinfectant than chlorine and is relatively independent to pH. As a result, the required CT value tends to be much lower for chlorine dioxide than for free chlorine (TCEQ, 2013).
There are several other benefits, such as enhancing CxT disinfection credits when chloramines are used as the secondary disinfectant, and from chlorine dioxide’s key DBP, the chlorite ion, entering distribution at a low sub-MCL limit for control of nitrification processes and extension of the chloramine life in the distribution systems. Chlorine dioxide can also be surprisingly cost effective relative to alternative disinfectants in many use cases.
For more information on these benefits, please view our webinar entitled “A Competitive Review of Pre-oxidants.”
Getting Started
Getting started is fairly straightforward. The utility will work with Evoqua’s qualified service personnel to perform a demand study on raw water samples. A baseline chlorine dioxide dose will be determined to meet requirements for oxidizing dissolved organics, iron and manganese in advance of downstream chlorination, and/or achieving log inactivation (CT) credits for disinfection.
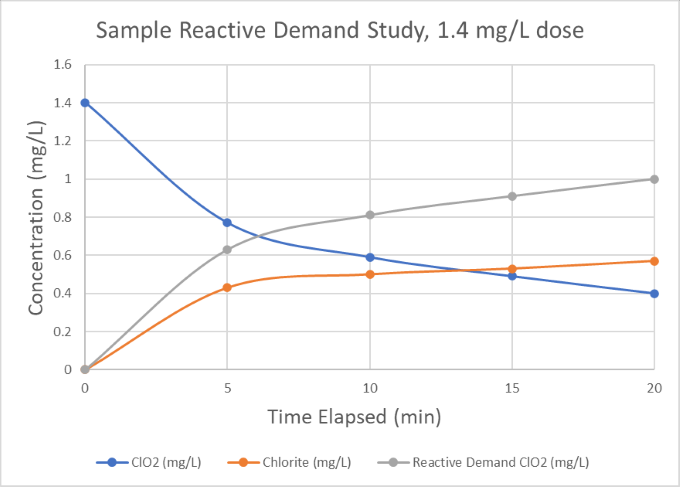
Once the evaluation is complete, the chlorine dioxide generator will be sized according to the required dose and plant flows.* For example, from the demand study below we can see that a 1.4 mg/L chlorine dioxide dose in the raw water samples was consumed down to 0.4 mg/L after 20 minutes, indicating a 1.0 mg/L reactive demand for a 20-minute residence time. As such, we would consider a baseline chlorine dioxide dose of 1.0 mg/L and size the generator to have suitable excess capacity to account for increasing plant flow or contamination of the water supply.
Evoqua will work with the plant to determine the optimal type of generator and reaction chemistry. After the system is manufactured, Evoqua typically completes the install, startup and commissioning within 3-5 days of delivery to the site.
Operator Training
We recommend that new users of chlorine dioxide undergo a 3-day training program instructed by one of our dedicated service staff. During the training operators learn how to navigate the controls, tune the generator manually in the event of electronics failure and perform maintenance checks. We also provide thorough training on the chemistry and safety considerations when working with chlorine dioxide.
Safety Considerations
On the topic of safety, what should an end user or engineering firm know in order to ensure safe operation of chlorine dioxide in the plant?
Like any oxidizer, working around and handling chlorine dioxide and its starting chemical raw materials (precursor chemicals) requires the use of appropriate PPE with safety glasses, steel toes, rubber or nitrile gloves, etc. As with all chemicals, it requires respect. The operation of the equipment is safe, given proper training and an understanding of the generation process and properties of the starting chemical raw materials.
Evoqua’s Millennium III™ generators are built to dissolve a concentration of produced chlorine dioxide gas immediately into water, and to prevent over-concentration of the chlorine dioxide solution produced. We deem the maximum permissible concentration of delivered aqueous chlorine dioxide solution from these generators to be 3000 ppm, and to be operated under vacuum conditions. Motive water is pumped at a constant rate and constant pressure across the generator’s vacuum eductor to induce precursor chemical flow into the reaction column in controlled quantities to ensure that this concentration limit is not exceeded.
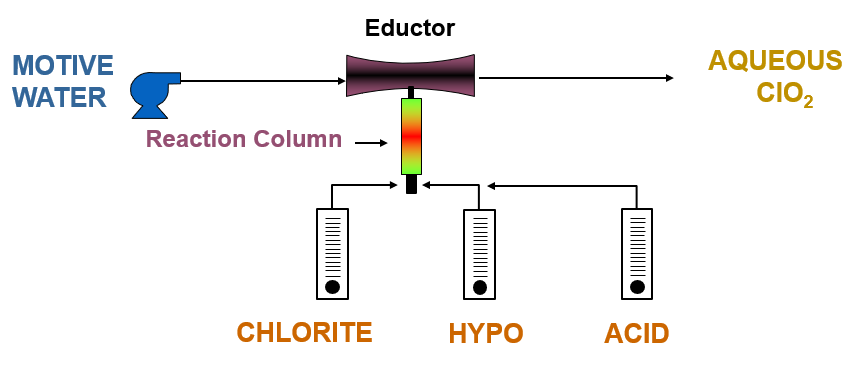
Generation of chlorine dioxide using 3-chemical sodium chlorite, bleach, and hydrochloric acid chemistry.
Chlorine dioxide is very water soluble (more so than chlorine gas), and even at the max 3000 ppm concentration (equivalent to 0.3% by wt. and 99.7% by wt. water), the risk of degradation is low unless concentrated chlorine dioxide gas in air is exposed to a high temperature environment under high pressure conditions. In general, we recommend that the system is stored in an environment no greater than 100°F, with safety factors built in.
Additionally, the system has fail-safe pneumatically operated valves if motive water flow, power or vacuum is disrupted, which shuts down the reaction and the system entirely. This well-established technology further limits risk.
Routine Maintenance
Like any piece of equipment involving pumps, valves and instrumentation, we recommend biweekly visual inspections. In practice, maintenance is typically advised for monthly cleaning of critical components, such as solenoid valves, flow meters, check valves and strainers. This may take a few hours once the operator is familiarized with the system.
Quarterly maintenance often consists of lubricating pump inlets and outlets, pressure regulator cleaning and lubing, replacing discolored tubings, and most critically, inspecting the nozzle and throat of the vacuum eductor that draws the precursor chemicals. There are some additional cleanings and part replacements as necessary, but maintenance is comparable to most other treatment equipment. We also recommend evaluating whether the system is generating chlorine dioxide optimized for your requirements.
The entire quarterly maintenance process may take a full day per generator for flow pacing systems, and a half a day for the manual systems. With a full-service package from Evoqua, our trained service team can handle all the heavy lifting, so the operator can focus on keeping the plant running.
Chemical Storage & Sourcing
There are a lot of known chemistries for generation of chlorine dioxide. In general, we recommend two main chemistries for water treatment plants due to their 95%+ yields: either 2-chemical chlorine gas and sodium chlorite chemistry, or 3-chemical sodium chlorite, bleach and hydrochloric acid chemistry.
Other chemistries tend to either be non-economical for the required generation rates or else consume immense amounts of energy contributing to higher OPEX. The trend is that many facilities have been converting to 3-chemical lately with the intention to avoid chlorine gas and we anticipate the 3-chemical chemistry to surpass the chlorine gas chemistry in the coming years.
As far as sourcing goes, chlorine gas, 12.5% sodium hypochlorite and 15% hydrochloric acid are all readily available from the big-name chemical suppliers. 0.8% on-site hypochlorite generation systems, or OSEC® weak bleach generators are also readily available through Evoqua and are operational for the 3-chemical systems. As for sodium chlorite, this can be sourced through Evoqua under our trade name, Akta Klor 25, a 25% sodium chlorite solution. 31% sodium chlorite solution is also available.
Reducing Workload
There is a growing trend indicating a decline of qualified workers in the water industry as more and more operators are reaching retirement age. We have found that chlorine dioxide can potentially reduce the workload for operating the plant in some circumstances.
In situations where utilities are consistently struggling to keep within MCL limits of THMs and HAA5s, ClO2 greatly reduces the repercussions of managing paperwork and reporting nonconformances to the EPA. The same goes for meeting log reduction CT credits.
That being said chlorine dioxide does require more maintenance than say a chlorine gas feeder, and oftentimes are even dosed in tandem to maximize synergy of THM reduction. Thus, in these instances there may be a small increase in labor requirements. On the other hand, enhanced pre-oxidation on the front end will assist in settling and can help extend the life of filter runs, which can save substantial labor in the plant.
The decline of qualified workers in the water industry and the general shortage of workers across America is certainly alarming but fortunately there are ways to limit the impact on your future operations. In general, we recommend leveraging a full-service program for your operations wherever possible. This not only places the operation of equipment and maintenance in the hands of the experts, ensuring equipment longevity, optimization of dose rates and associated cost savings, but also eases the demand for tedious labor from increasingly stretched operators.
EPA Sampling & Reporting
The implementation of chlorine dioxide requires changes to the utility’s EPA sampling & reporting plan. Measurements of chlorite and chlorine dioxide are required to satisfy regulatory requirements, but also to ensure that your system is maintaining efficient operation.
Chlorite is a degradation product of chlorine dioxide and requires monitoring & reporting. Daily monitoring is required at the distribution system entry point and monthly samples are required at three locations at the point of first customer, at the average residence time and at the maximum residence time. Compliance is based on the average of these three chlorite monitoring locations each month and the maximum contaminant level (MCL) is required to be under 1.0 mg/L. Additionally, chlorine dioxide residual has a maximum residual disinfectant level (MRDL) of 0.8 mg/L and compliance is based on daily sampling at the treatment plant (EPA Office of Water, 2010).
Stage 2 DBPR Regulated Contaminants
| Regulated Contaminant | MCL (mg/L) | Byproduct of | MRDL (mg/L) |
|---|---|---|---|
| TTHM | 0.080 | Chlorine | 4.0 (as Cl2) |
| HAA5 | 0.060 | Chlorine | 4.0 (as Cl2) |
| Chlorite | 1.0 | Chlorine Dioxide | 0.8 (as ClO2) |
The daily sampling can be achieved by a number of methods; however, it is recommended to utilize the Kemio®** disinfection residual monitoring kit which utilizes an EPA approved method for measurement of chlorite and chlorine dioxide residuals. The utility then maintains data entry of these specimens on a daily reporting form.
The monthly samples from the three monitoring locations are required to be analyzed by a certified 3rd party approved by the state (EPA Office of Water, 2020) with the capability of performing one of the EPA-approved test methods for the respective analyte (EPA Office of Water, 2019). These 3rd party testing labs will typically send a sample kit with instructions at the beginning of the month, but utility involvement is often limited to collection of the samples and shipping them on ice to the lab, who will likely provide the results within 5-7 business days upon receipt of the samples, dependent on the type of testing performed. The utility would then submit the results to the state.
Mitigating Risk
As we discussed earlier, use of chlorine dioxide requires reporting of chlorite concentrations to the EPA. The reason for this is that the chlorite ion itself has a MCL of 1.0 ppm and is a degradation product of chlorine dioxide as it is consumed by organics, iron and manganese, and microbials.
In general, approximately 70% of the chlorine dioxide dose applied to surface water will revert to chlorite ion. This means that a maximum dose of 1.4 ppm is permissible of chlorine dioxide unless a chlorite ion reduction strategy is incorporated to quench the chlorite such as dosing ferrous ion as ferrous sulfate or ferrous chloride solution ahead of coagulation & flocculation or adding a GAC cap on a multimedia filter.
For example, if a residual is desired for obtaining CT credits, a dose of up to 2.0 ppm is often necessary to achieve a measurable residual ahead of coagulation and flocculation of around 0.5 to 1.0 ppm. As such, chlorine dioxide dose requirements need to be carefully determined and should ideally be linked to current plant flow and reaction demand to ensure that the concentration of chlorite will not be exceeded without a removal strategy.
Evoqua offers residual monitors and kits to detect disinfectant residuals to assist in planning, but as long as the generator is sized correctly the risk of noncompliance due to chlorine dioxide or chlorite residual is very small.
Another risk is the off-gassing of chlorine dioxide, whether in generation or at the dosing point. Inhalation of vapors should be avoided, and adequate ventilation is recommended in the generator room, especially for batch systems in which chlorine dioxide solution is held in a vented batch tank. We typically advise that the batch tank is vented to the outdoors to mitigate vapors from escaping into the generator room. As an added safety feature, we recommend a chlorine dioxide gas monitor to detect presence and concentration of chlorine dioxide and chlorine gas in the ambient surroundings. In direct-feed generator systems that dose directly to the treatment point, there is minimal risk of off gassing as the solution is contained in the piping until the discharge point.
Implementation Results
Results are variably dependent on treatment objectives, current treatment approaches, the dosed chlorine dioxide concentration, and of course raw water characteristics, etc. To be conservative generally a typical implementation of a 1.0 ppm pre-oxidant dose can achieve a reduction in TTHM between 20%-50%. As it pertains to achieving Stage 2 DBPR compliance, even a 20% TTHM reduction is typically more than enough to achieve compliance to 80 ppb on average considering most violations are below 100 ppb.
For iron and manganese, we would likely see a reduction by over 50% and with shorter retention times than chlorine while simultaneously reducing the risk of DBP’s.
As for those looking to achieve log inactivation credits, the required CT factor for chlorine dioxide represents a reduction of roughly 70% for giardia cysts relative to chlorine, so this can drastically shorten retention times and with controlled residual management can greatly reduce the risk of biofilm in distribution, especially with the use of chloramine residual.
Conclusion
Overall chlorine dioxide works well for many situations. Keep in mind that regardless of the expected benefits, it should be evaluated among several treatment alternatives and used as part of a multibarrier treatment approach.
If you have any specific questions, please reach out to Glenn and/or Nick using their contact information listed below.
About the Authors

Glenn Holden

Nick Swanson
References:
EPA Office of Water. (2010, August). Comprehensive Disinfectants and Disinfection Byproducts Rules (Stage 1 and Stage 2): Quick Reference Guide. Retrieved February 22, 2022, from https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=P10058CG.txt
Analytical Methods Approved for Drinking Water Compliance Monitoring under the Disinfection Byproduct Rules. Retrieved February 22, 2022, from https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=P100WD1L.txt
* Evoqua’s Chlorine Dioxide Generation systems make no claim regarding disinfection. These systems apply EPA-registered chlorine dioxide precursor chemicals, for which the generation of chlorine dioxide used as a disinfectant for microbial control in water is presented on the labels’ directions for use. Performance limitations depend on feed conditions, overall installed system design, and operation and maintenance processes; please refer to Operations Manuals. For more information: Contactus@evoqua.com
** Kemio® is a registered trademark of Palintest LLC.
